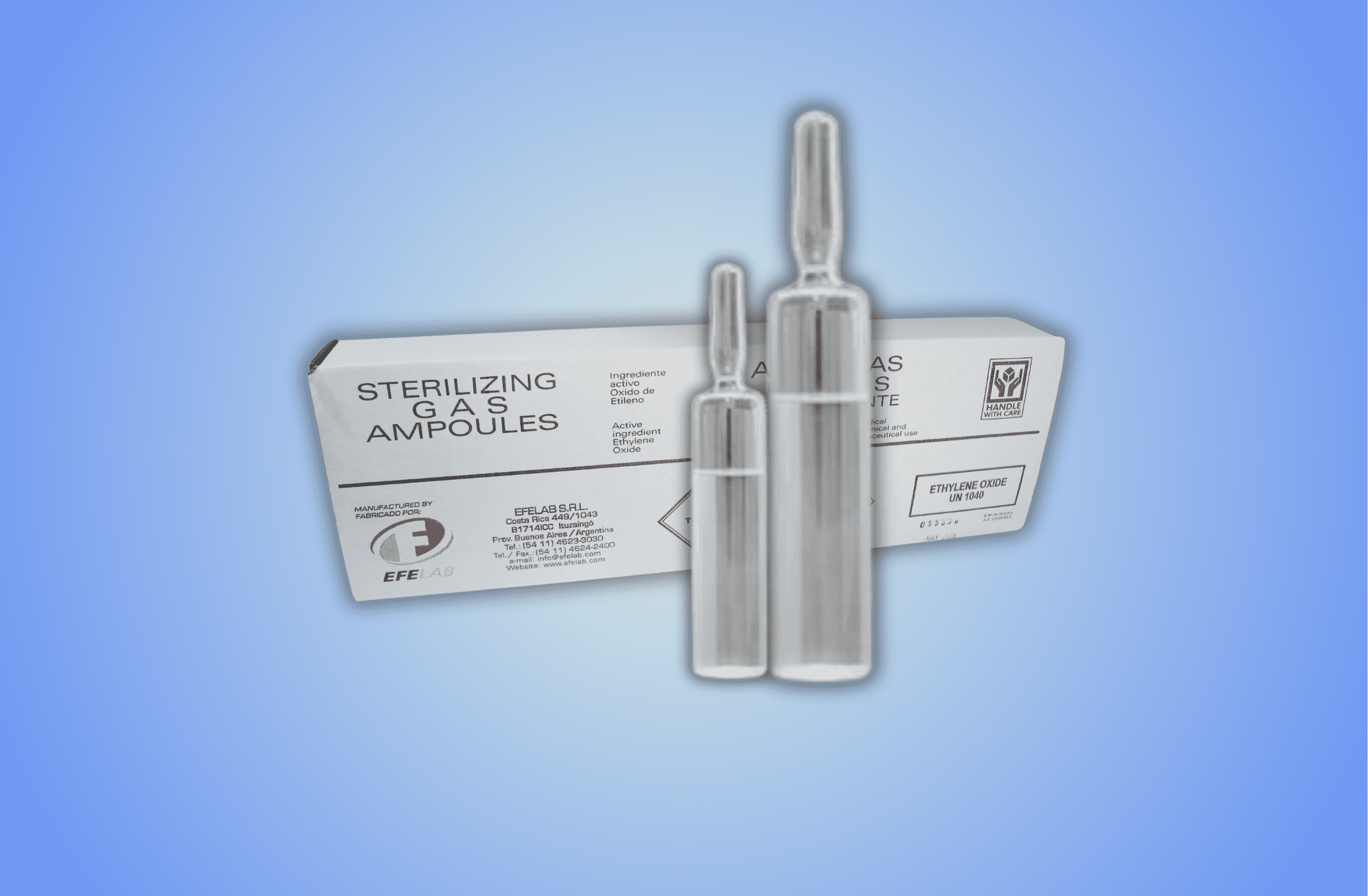
Ethylene Oxide Cartridges
Our company manufactures and markets 100% pure ethylene oxide cartridges. It should be emphasized that these cartridges have stringent quality control standards on materials, contents and weight throughout manufacturing and packaging. Product evolution follow-up is carefully made on a unit-per-unit basis.
Ethylene Oxide Cartridges:
- Pure Ethylene Oxide.
- Bactericide, Viricidal and Fungicide Action.
- Airtight, Inviolable and Disposable.
- CFC Free (Freon). Does not affect the Ozone Layer.
- Safe for plant personnel.
- Within a sterilizer it allows to effectively sterilize when all the critic parameters are controlled – EO gas concentration, exposition time, temperature and relative humidity.
Applicable to many different sterilizer types and brands available in market:
- 3M Sterivac® – Amsco® – Hogner® – Del Giudice® – Quetzal® – Mazden® – BioMedica® – Mazzarotti® – Among many others.
Compatibility table for sterilization equipment using the following capsules:
| CODE | DIAMETER | HEIGHT | CAPACITY IN GRAMS |
| 1007 / 10 | 25 mm | 95 mm | 7/10 gr |
| 1020 | 25 mm | 105 mm | 20 gr |
| 1025 / 30 | 25 mm | 105 mm | 25/30 gr |
| 1067 | 35 mm | 105 mm | 67 gr |
| 1070 | 35 mm | 140 mm | 70 gr |
| 1100/3 | 38 mm | 165 mm | 100 gr |
| 1128/3 | 38 mm | 165 mm | 128 gr |
| 1134 / 3 | 38 mm | 165 mm | 134 gr |
| 1130 | 50 mm | 133 mm | 130 gr |
| 1170 / 3 | 45 mm | 176 mm | 170 gr |
| 1160 | 50 mm | 174 mm | 160 gr |
| 1180 | 50 mm | 174 mm | 180 gr |
| 1200 | 50 mm | 174 mm | 200 gr |
| 1220 | 50 mm | 174 mm | 220 gr |
| 1230 | 50 mm | 174 mm | 230 gr |
| 1250 | 65 mm | 260 mm | 250 gr |
| 1370 | 65 mm | 260 mm | 370 gr |
Why choosing us? An approach based on product safety and quality:
Our company manufactures ethylene oxide cartridges with the highest quality standards in the market, being the first and only company in Argentina to have a quality management system certified in ISO 9001: 2015 and ISO 13485: 2016.
We only use pure ethylene oxide in medicinal quality (purity of analytical grade) and suitable containers for optimum gas conservation.
We carry out exhaustive weight controls and rigorous sealing controls on each unit produced.
The process is systematically monitored as part of our registration system, which guarantees the safety and efficacy of the product, providing also predictability to the user regarding the performance of the product during the sterilization cycle.
The containers are placed in boxes that comply with international quality standards for the correct preservation of the product and specifically comply with United Nations regulations for the safe transport of dangerous goods (UN mark). These tests consist of tests of fall, resistance to compression and absorption of water that are carried out periodically on the boxes containing the containers inside. Said tests are carried out in the I.N.T.I. (National Institute of Industrial Technology), an official testing and control body, and reviewed and approved by P.N.A. (Naval Prefecture Argentina).
All these aspects result in a higher quality and safety for the users and assure an optimal performance of the product during the sterilization cycle.